Viedoc solves the data challenges of clinical trial professionals by improving how sites, contract research organizations, sponsors, and even trial participants share and manage sensitive data.

"One of the best things about Viedoc EDC is that it is very user-friendly and easy to use. One can navigate around Viedoc with relative ease. Also, customer support provides replies within 1 working day."
— Richard M.

"Viedoc is a very user-friendly platform. In fact, I prefer it over other competitors. Easy to navigate through and has very good features. The user training provided is quite simple to understand."
— G2 verified user
Viedoc combines an intelligent core with a streamlined interface, at-a-glance dashboards, and powerful tools that offer real-time metrics. Our unified system is carefully designed to provide reliable access to trial data from any device.
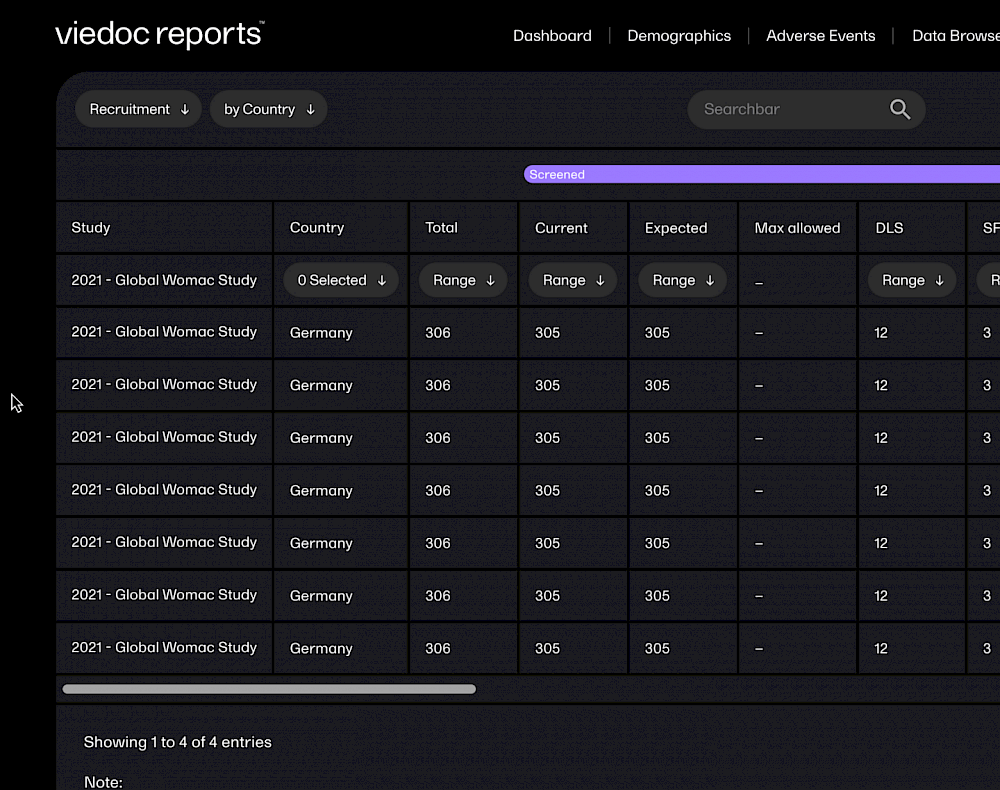
With Viedoc, you can get your study build started in minutes, with ready-to-use, tailorable templates. Viedoc is fully web-based, so requires no client installation. You can even receive data directly from trail subjects via their own devices.
With Viedoc, you’re free to manage and configure all kinds of settings instantly—from design elements to user permissions. Viedoc’s intuitive design tools and CDISC CDASH templates enable you to adjust your data without interruptions or loss.
Move seamlessly between a complete set of applications and features that cover all your needs—from study setup to data delivery.
Get all the tools you need for your decentralized clinical trials and improve the subject experience.
Our eClinical suite is 50% faster than other systems—from study setup to UAT-1.
New updates are released regularly, and all are backward-compatible. No additional system validation is required for new releases.
Make mid-study changes at all levels with no system downtime in a self-service fashion.
Pay as you go. There are no license fees until after the study starts, and no unexpected charges.
Access regional support and infrastructure, ensuring maximum performance wherever you choose to run your study.
All data is safeguarded using high level security measures, including robust backup systems, advanced data encryption, and audit trails of all activity.
Full documentation that meets inspection requirements, updated with every release.
Get started right away with our intuitive online guides and upskill your team with advanced study builder training.

"Data capturing has been made easy because of viedoc, it is incredibly robust and easy to navigate"