
Case Study

Case Study

Case Study
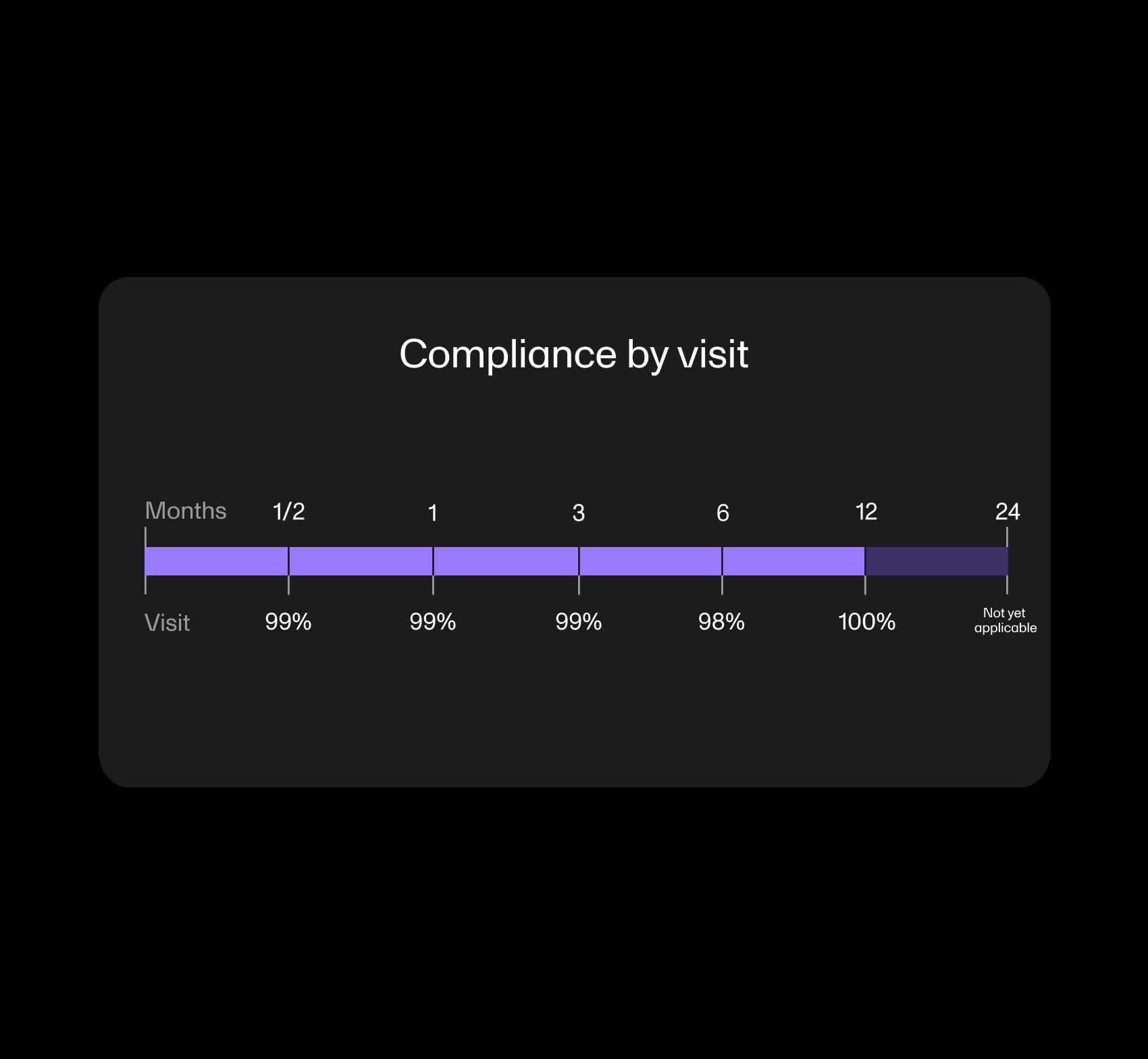
Case Study

Case Study

Reports
/two-female-doctors-ipad.png)
Case Study
/doctor-smiling-patient.png)
Case Study

Webinars

Webinars

Webinars
/woman-man-working.jpg)
Guide

Guide

Reports

Webinars
/case-study-how-viedoc-adds-value-to-tecro-research-and-lmus-tb-trial.jpg)
Case Study

Case Study
/case-study-why-qmed-chose-viedoc-for-medical-device-trials-data.jpg)
Case Study
/niche-case-study-exploring-the-potential-of-psychedelics-for-mental-health.jpg)
Case Study
/a-case-study-on-how-link-utilized-viedoc-powered-screening.jpg)
Case Study
/american-biometric-trials-a-case-study-on-linical-and-viedocs-success-in-the-us.jpg)
Case Study
/how-a-nordic-cro-uses-viedocs-adaptable-platform-to-manage-studies-in-brazil.jpg)
Case Study
/how-a-powerhouse-cro-makes-clinical-trials-a-peoples-business-with-viedoc.jpg)
Case Study
/why-the-kondo-method-means-more-competitive-risk-based-monitoring.jpg)
Case Study
/how-scandinavias-largest-hospital-uses-viedoc-to-evolve-healthcare.jpg)
Case Study
/how-this-berlin-based-cro-uses-viedoc-in-imaging-studies.jpg)
Case Study
/international-cro-stays-ahead-with-viedocs-modern-clinical-trial-technology.jpeg)
Case Study
/prepare-for-greater-discoveries-from-the-university-of-montpellier.png)
Case Study
/how-a-world-leading-cro-in-america-praises-viedocs-capabilities-to-simplify-clinical-trials.jpg)
Case Study

Webinars

Webinars

Webinars

Webinars
Get the latest Viedoc insider tips and EDC industry trends direct to your inbox. Sign up for our newsletter, and don’t miss the latest updates and insights.