Clinical trial record sharing solution
Streamline document and asset sharing with clinical personnel and study participants
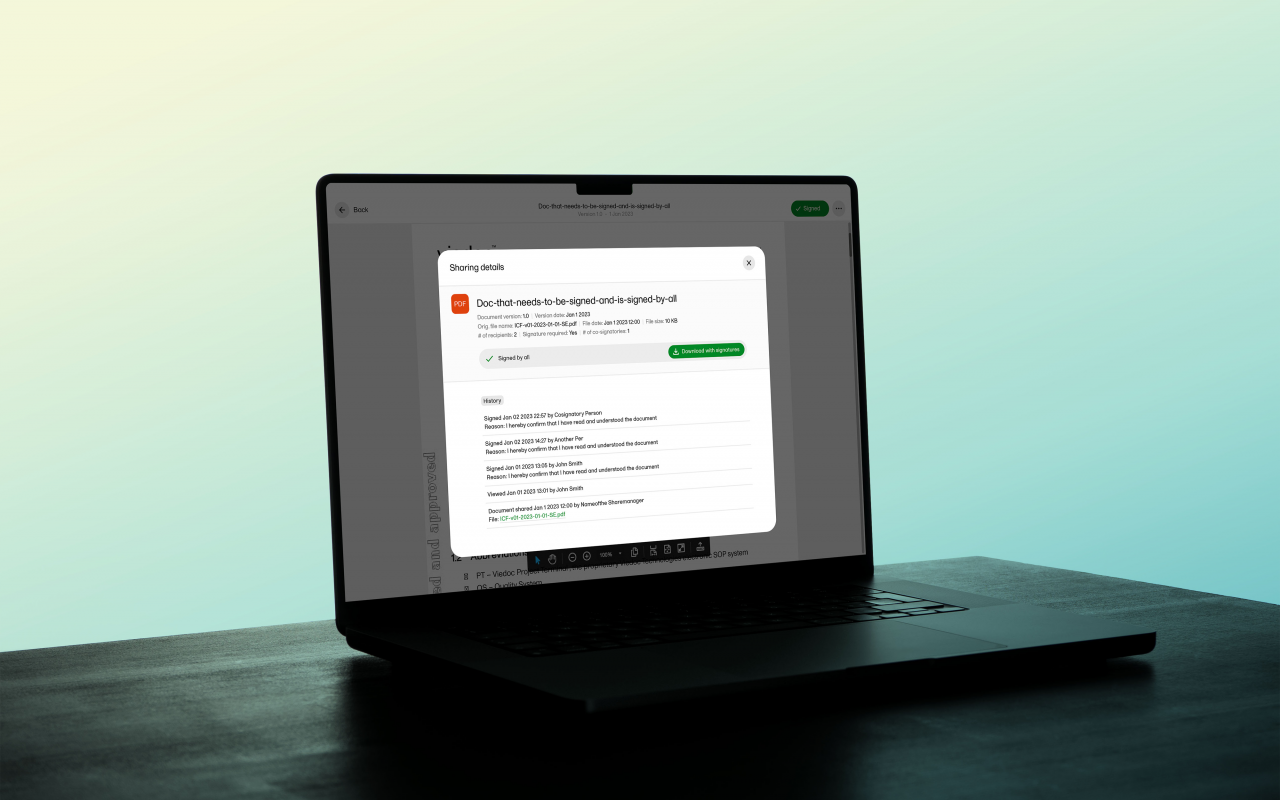
Viedoc Record Sharing enables our platform users to share study data with any other authorized users. Working within our comprehensive eClinical suite, this solution makes it quick and easy to share not only documents, but images, videos, reports, and more. Record sharing is included at no additional charge.