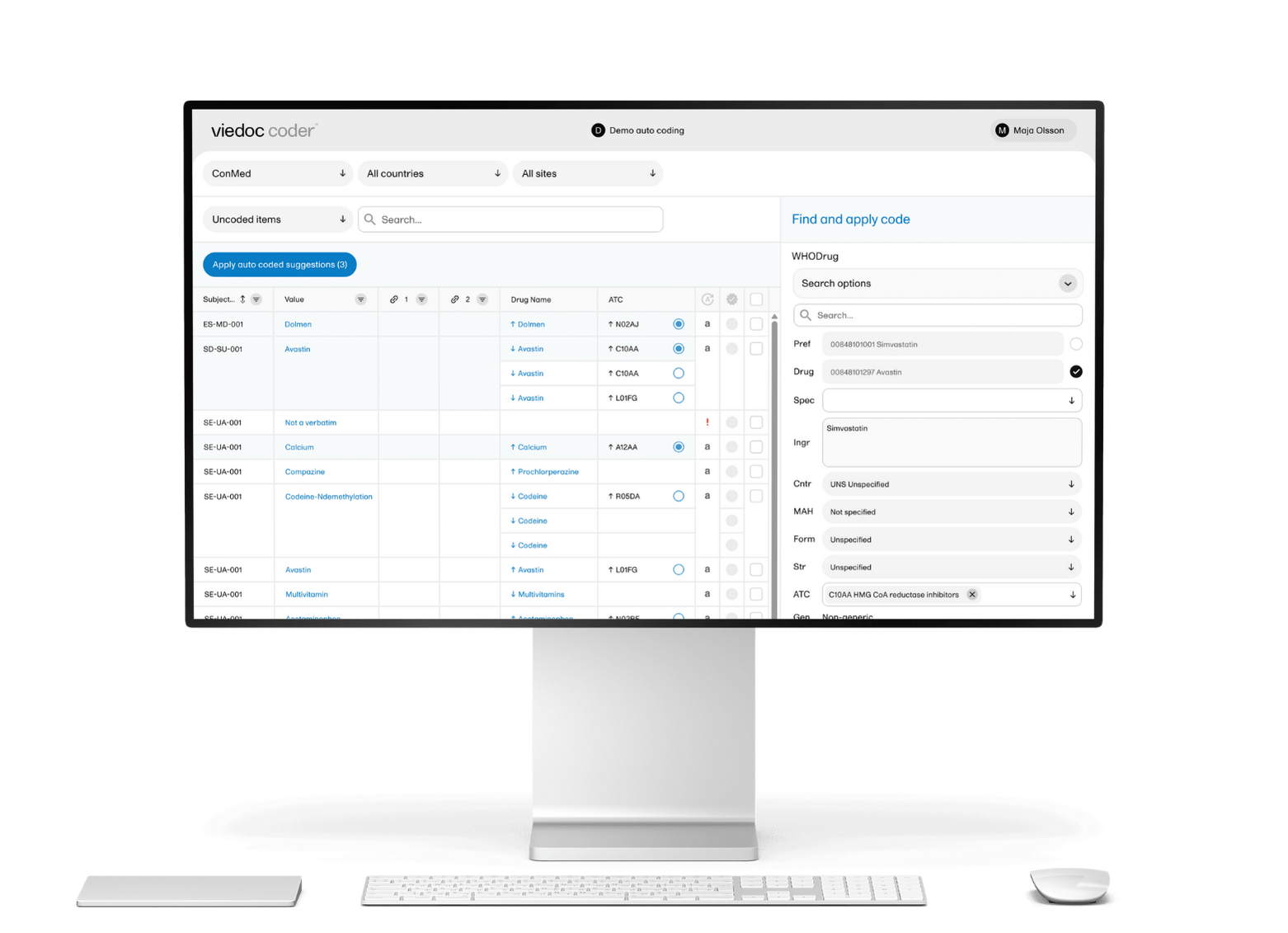
December 03, 2025

December 03, 2025
/female-doctor-with-tablet.jpg)
September 18, 2025
/SDL/sdl-woman-laptop-ui.png)
August 14, 2025
July 31, 2025
/laughing-doctor-patient-laptoop.png)
July 17, 2025
/doctor-patient-laptop-focused.png)
June 30, 2025
June 25, 2025

June 19, 2025
/clinician-patient-ipad.png)
June 18, 2025

June 16, 2025
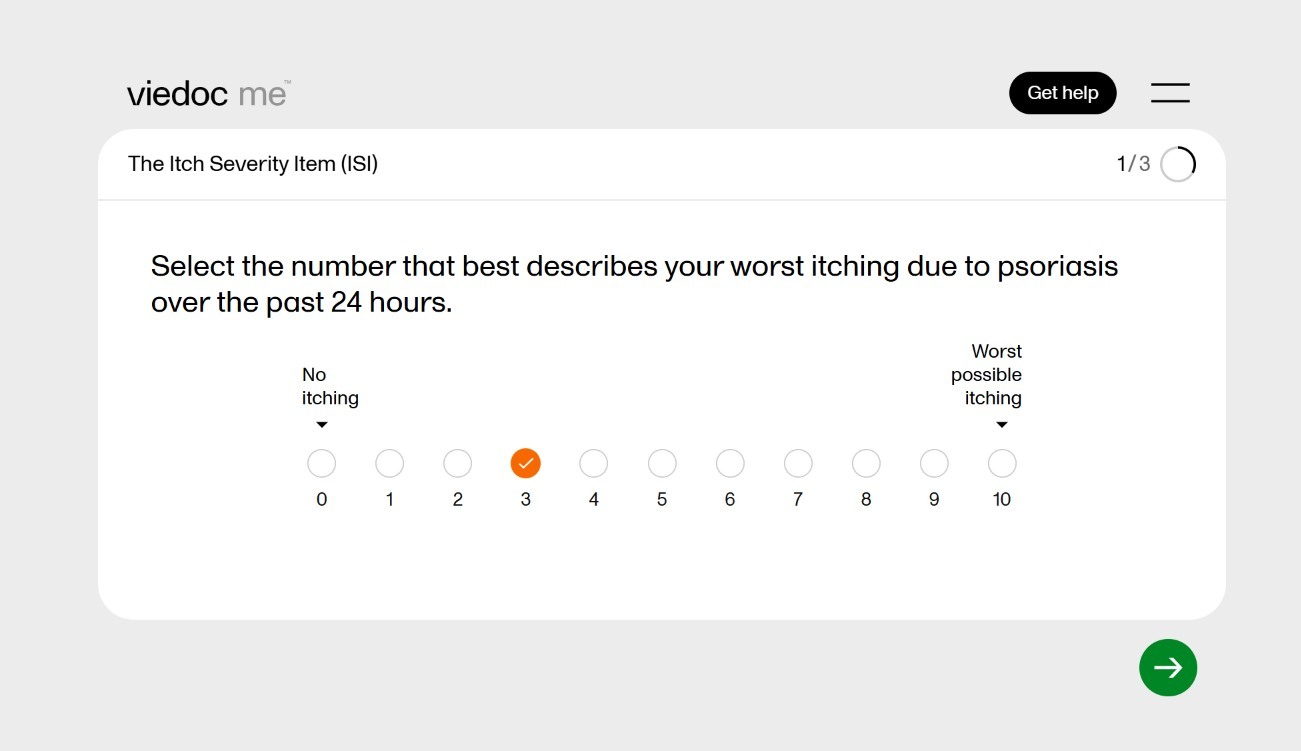
June 12, 2025