What are the different types of EDC?
EDC systems can be categorized according to their deployment and data storage methods. Common types include: cloud-based EDC, on-site EDC, hybrid EDC, web-based EDC, and integrated EDC.
What is the best EDC system for clinical trials?
There isn't one best EDC system. The best system for your clinical trial will depend on your requirements, budget, and the level of support needed.
What export formats are supported?
Viedoc supports exports to Excel, ASCII, SAS, PDF, and CDISC ODM.
Is Viedoc CRF 21 CFR Part 11 compliant?
Yes, statements of compliance with various regulations, including 21 CFR Part 11 and data privacy laws, are available upon request.
Does Viedoc store an independent contemporaneous investigator copy of the eCRF?
Yes, all versions of a form are stored at the time the form is saved, and these remain under the investigator's control.
Where and how frequently are backups performed?
Backups are taken, encrypted, and replicated to the paired Microsoft Azure region every five minutes. Additionally, a full backup for each instance is encrypted and transferred to cold storage at a third location, where it is read back, restored, and tested for integrity every 24 hours.
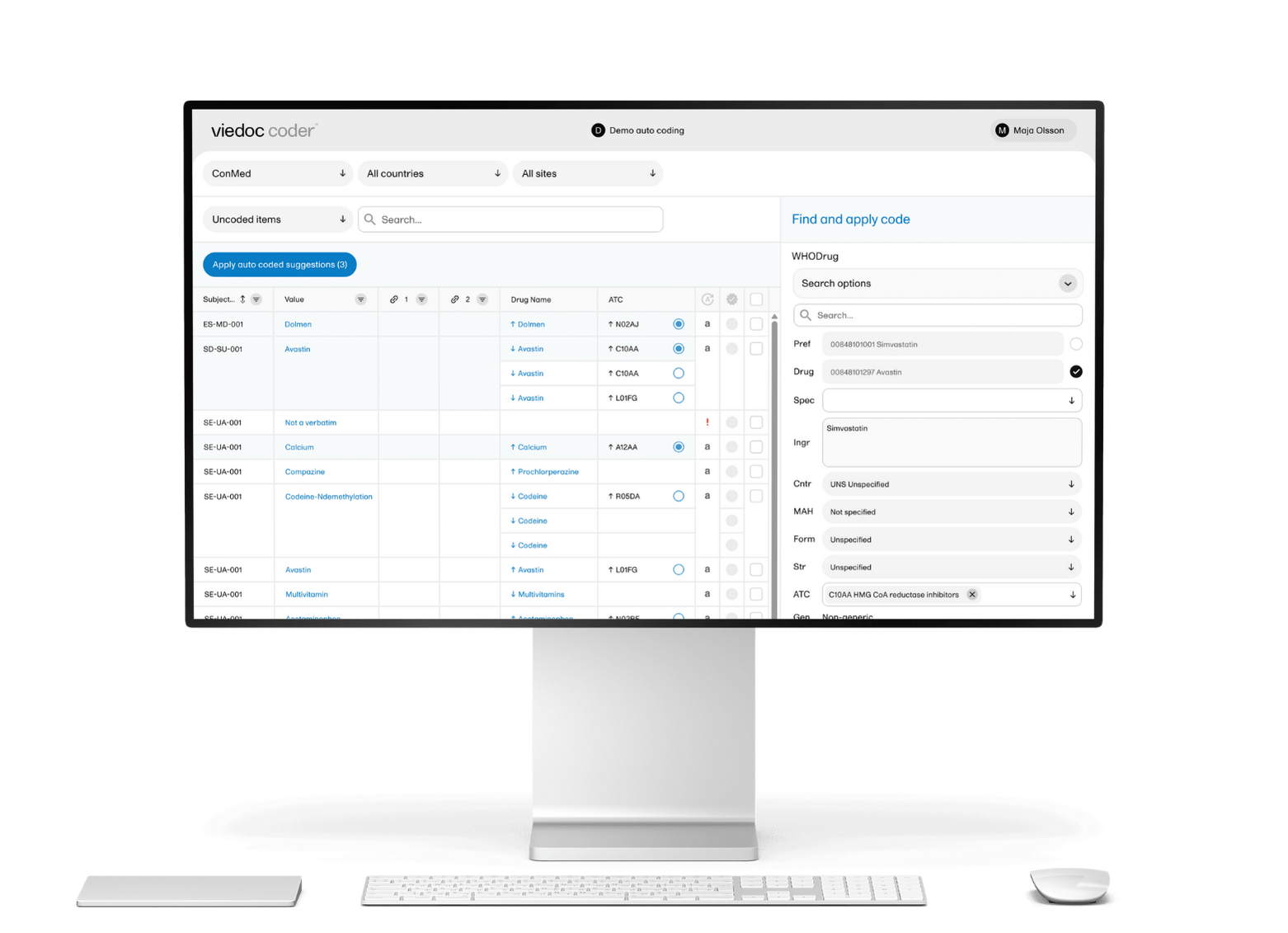
Can Viedoc EDC handle complex edit checks?
Yes, edit checks can be configured to compare multiple data points across different forms and events. There is no limit to the number of data points or operations included in the validation logic. This ensures greater reliance on system validation checks and reduces the need for manual offline efforts.
How does Viedoc address interoperability challenges for organizations using multiple software vendors?
With rich REST API and integration capabilities, Viedoc EDC enables seamless connectivity and compatibility with any software.






/new-year.jpg)
/female-doctor-with-tablet.jpg)