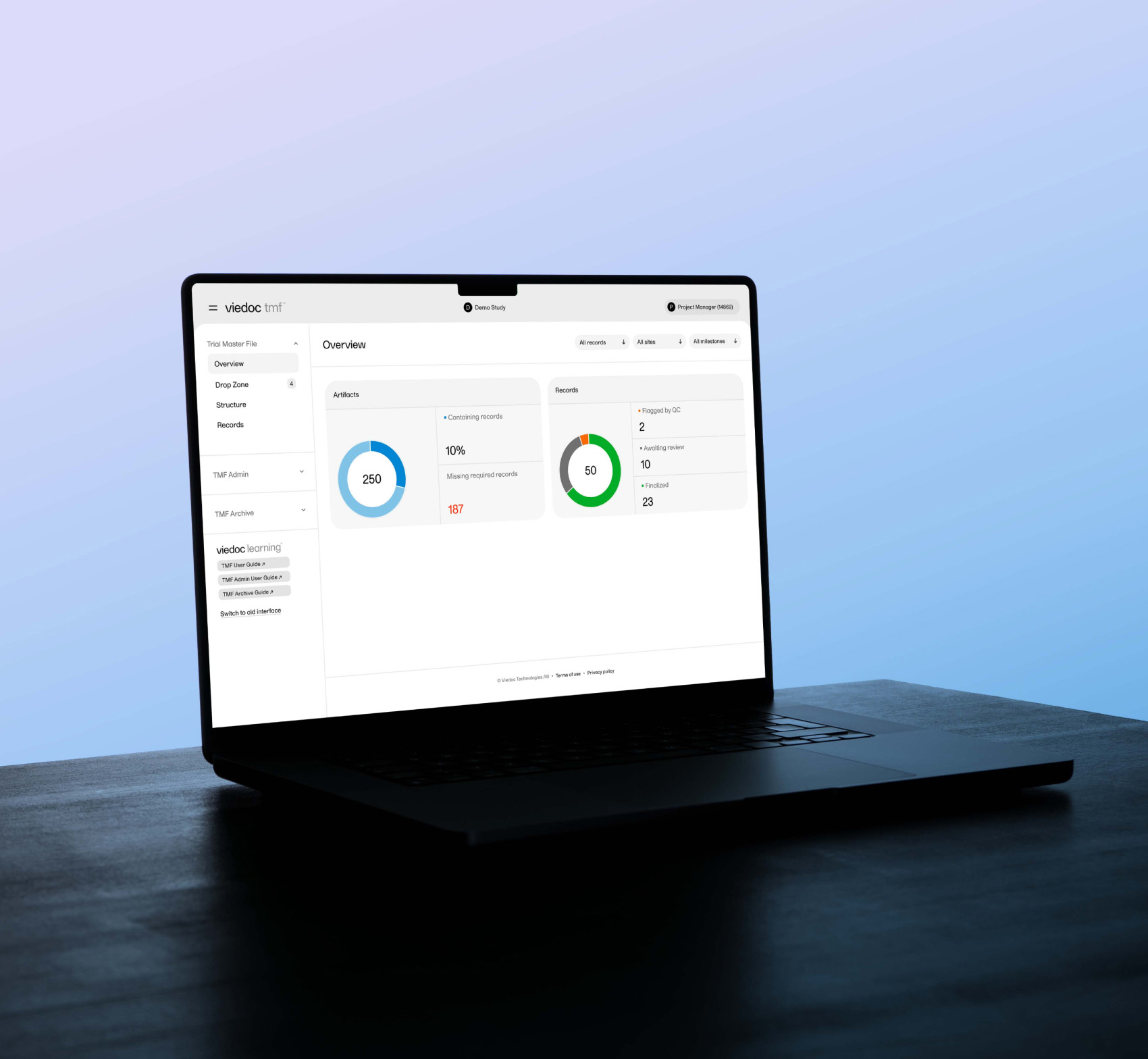
EDC clinical trial software
Flexible, intuitive, and secure EDC management system
Viedoc EDC helps users easily collect, validate and report on clinical trial data. With our browser-based interface, you can capture and manage data with greater accuracy and efficiency. We’ve made our EDC software flexible, effortless, and dependable, so you can focus on producing more accurate outcomes.











/woman-man-working.jpg)